Would you like to know how essential oils work on a chemistry level? How molecules interact with one another? Whether you’re here for your own curiosity or for a better understanding of the product you’re using, you came to the right place.
here for your own curiosity or for a better understanding of the product you’re using, you came to the right place.
Essential oils consist, of a surplus amount of chemicals that contribute to the aroma and therapeutic properties in the oils.
Your essential oil all started at the root, the root of a plant that is. These plants give off an aroma to protect themselves from organism such as:
- Predators
- Bacteria
- Pests
- Fungi
I’m more than happy to share my connection with chemistry, and more importantly, lower back pain. In my article I will cover:
- The basics of essential oils
- Functional groups
- The molecular structures
Let me take you behind the scenes of the chemistry of essential oils.
Starting With The Basics
You can’t understand a chemical without an element, you know that letter in the periodic table?
the Elements and molecules found in essential oils are:
- Oxygen
- Hydrogen
- Carbon
A mix of these elements – known as a compound can be found in the oils. Each varies in:
- structure
- solubility
- rate
- evaporation
- therapeutic properties
Essential oil for lower back pain are extracted from the same species, but each plant varies in their chemical composition – known as chemotypes. It’s vital to know the chemotypes of essential oils in aromatherapy…after all, you do want to know what you’re inhaling right?
Here is an example:
Ex: Let’s take the plant Basil and its essential oil. The main composition of “exotic basil” is called “methyl chavicol”, a compound that is toxic and possible carcinogenic, and it shouldn’t be used in aromatherapy. See why you shouldn’t? It’s not safe whatsoever.
But for other uses like in a “Linalol” chemotype, knows as French or true sweet basil, its more preferred.
That brings me to the suppliers… The reason you get the right chemotype is that suppliers have to state which chemotype they’re selling…which brings me to taking hydrogen…
Hydrogen to Hydrocarbons
What happens when you mix a carbon atom with a hydrogen atom? You get this super transformer. And an even better name to associate with – Hydrocarbon!
Hydrocarbons are the simplest of organic compounds.
Do you know we use hydrocarbons every day already? We don’t see them but they’re out there. They come in the form of:
- fuels
- candle Wax
- oil
- gasoline
- LP gas
- natural gas
hydrocarbons are the ground level starting materials in synthesizing consumer products like:
- soaps
- fabrics
- dyes
- cosmetics
- plastics
- drugs
- rubber
Rule 1: Classifications
Hydrocarbons are classified into four different types:
- Alkanes
- Alkenes
- Alkynes
- Aromatic Hydrocarbons
We’ll be focusing on aromatic hydrocarbons, but you can’t have them with the other 3. And you’ll soon see why.
Functional Groups
A functional group is a characteristic atom or group of atoms – that are a part of hydrocarbons. And a group of organic compounds that all have the same functional group is a family.
A family is just a name used to identify how the bonds of oxygen, hydrogen, and carbon all form together as a molecule.
Here are both a functional group and a family.
You have 7 families.
- alcohols
- ethers
- aldehydes
- ketones
- carboxylic Acids
- esters
- amines
And for each you have a general formula that shows you have they need to look. Your condensed formula is similar to what you see in an empirical formula:
For ex:
- H2O
- PF2
- MNO2
Instead, for a hydrocarbon you see something like:
- CH4
- CHOOCH
- C3N
And then you have the name they give it. A name goes more in depth, but easy to do once you grasp it. But for this article, I will keep that short.
Have you thought to ask yourself how these molecules bond together? It’s because of their polarity – meaning they have the positive and negative charges that attract each other.
So let’s start with each functional group
1. Terpenes
Terpenes are the largest class of molecules you find in an essential oil. They are identified by their suffix: -ene( one of the four types of hydrocarbons – Alkene)
In the following segment I’ll talk more about:
- structure
- solubility
- rate
- evaporation
- therapeutic properties
Not all essential oils are rich in terpene, each will vary depending on the amount their chemical reactions yield – or how far there reaction converts to a product in the making of an essential oil.
Volatile: the ones that do have rich terpene, are very volatile! Making it a great source for inhalation.
Evaporation rate: Teprentine molecules have a rapid evaporation rate, meaning they are the ones you smell first in an oil blend(or perfume).
Most terpenes produce a warm and stimulating effect on your skin, which is necessary to helping your muscle and joint pain.
Common terpenes you’ll find are:
- Pinene. Found in pine, juniper, nutmeg frankincense, fir
- Limorene. Found in just about 90% of all citric essential oils
- Chamazulene. Found in a German Chamomile
Alcohols
Like Terpene, Alcohol also has a suffix – ol.
Volatile: They are often less volatile than terpenes.
Their therapeutic properties are not as strong as terpenes, so they can be smelled more towards the middle to base of an essential oil.
Structure: Alcohols will possess antibacterial and anti fungal properties and are non-toxic. Also, they are uplifting and sedative, which are the most useful groups in aromatherapy. Some alcohols have analgesic and antispasmodic effects.
Common alcohols include:
- Geraniol. Found in citronella, geranium, and palmarosa
- Menthol. Found in peppermint(in most topical skin creams)
- Linalol. Found in Basil and Lavender
3. Phenol
Phenol, Like alcohol end with a suffix in –ol.
Evaporation and rate: Phenol do not evaporate very quickly
Therapeutic properties: A very strong aroma
Structure: Contain bactericidal properties and produce a warming and stimulating effect.
Volatile: Very Volatile
Solubility: Phenol are very irritating, meaning they should only be used in moderation on your skin.
Common phenol include:
- Thymol. Found in thyme
- Chavicol. Found in the West Indian bay
- Carvacrol. Found in thyme and marjoram
- Eugenol. Found in cinnamon and clove
4. Aldehydes
Aldehydes have the ending – al, or will have the name aldehyde in the name. They are a derivation of primary alcohols.
Therapeutic Properties. Smell stronger than alcohols. Generally, they have calming and sedative effects, as well as antibacterial properties.
Solubility: Cause possible irritation to your skin and mucous membranes. Therefore, essential oils with high percentages of aldehydes should only be used in moderation.
Common Aldehydes Include:
- Neral. Found in lemongrass, melissa, and may chang.
- Geranial. Found in lemon grass, melissa and may chang.
- Citronella. Found in cintronells and lemon-scented eucalyptus.
5. Ketones
Ketones are derived from their counter part alcohols, and end with the suffix in –one. There’s one exception. If they are “camphor”
Evaporation: High boiling points, meaning it takes more heat to evaporate.
Therapeutic properties. Very potent, minty camphoraceous odor.
Structure: Ease nasal congestion and will benefit your respiratory system. Another great benefit is that they heal your wounds. The most toxic essential oils in the world contain ketones. However, not all are toxic.
Common Ketones Include:
- Fenchone. Found in fennel.
- Jasmone. Found in Jasmine.
- Camphor. Found in Rosemary.
6. Esters
Unlike the other functional groups in essential oils, esters have two words, and the first words suffix is replaced with a -yl
Therapeutic properties: A fruity aroma.
Structure: Made from a combination of alcohols and acid(carboxylic acids). They generally have anti-inflammatroy, antispasmodic, analgesic, and sedative properties. Are non-toxic.
Common esters include:
- Benzyl benzonate. Found in jasmine, and ylang ylang.
- Linalyl acetate. Found in clary sage, lavender, and bergamot.
- Isobutyl angelate. Found in Roman Chamomile.
Then there were Oxides.
7. Oxides
Oxides are unlike the other functional groups, they actually keep the name of the molecule they were derived from, following with the word oxide.
Therapeutic properties: Oxides have the strongest odors of all the molecules, and they are responsible for giving essential oils their aromas even at low percentages.
Common Oxides Include:
- Rose Oxide. Found in geranium, and rose.
- Cineol. Found in eucalyptus, spike lavender, and rosemary.
- Menthofruan. Found in peppermint.
Now to the fun stuff!
Molecular Structures
Have you ever read a chemical compound and wondered what that looked like? I sure did, and that’s what took me down my path into general chemistry, and organic chemistry courses.
Here are what the essential oils you use for lower back look like:
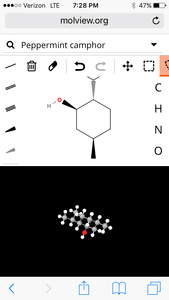
1. Peppermint
Molecular structure:
3D Model:
| Formula | C10H20O |
| Molecular weight | 156.269 u |
| Hydrogen bond donors | 1 |
| Hydrogen bond acceptors | 1 |
Percent composition
| C | 12.0107 u × 10 | 76.861% |
| H | 1.00794 u × 20 | 12.900% |
| O | 15.9994 u × 1 | 10.239% |
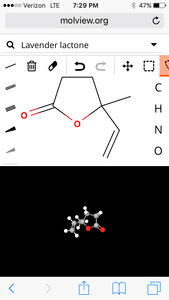
2. Lavender
molecular structure:
l
| Formula | C7H10O2 |
| Molecular weight | 126.155 u |
| Hydrogen bond donors | 0 |
| Hydrogen bond acceptors | 2 |
Percent composition
| C | 12.0107 u × 7 | 66.645% |
| H | 1.00794 u × 10 | 7.9898% |
| O | 15.9994 u × 2 | 25.365% |
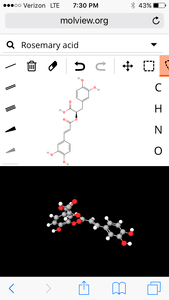
3. Rosemary
molecular structure:
3D Model:
| Formula | C18H16O8 |
| Molecular weight | 360.318 u |
| Hydrogen bond donors | 5 |
| Hydrogen bond acceptors | 8 |
Percent composition
| C | 12.0107 u × 18 | 60.001% |
| H | 1.00794 u × 16 | 4.4758% |
| O | 15.9994 u × 8 | 35.523% |
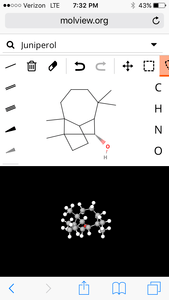
4. Juniper
molecular structure:
3D Model:
| Formula | C15H26O |
| Molecular weight | 222.372 u |
| Hydrogen bond donors | 1 |
| Hydrogen bond acceptors | 1 |
Percent composition
| C | 12.0107 u × 15 | 81.020% |
| H | 1.00794 u × 26 | 11.785% |
| O | 15.9994 u × 1 | 7.1951% |
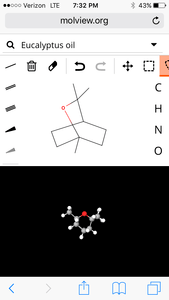
5. Eucalyptus
molecular structure:
3D Model:
| Formula | C10H18O |
| Molecular weight | 154.253 u |
| Hydrogen bond donors | 0 |
| Hydrogen bond acceptors | 1 |
Percent composition
| C | 12.0107 u × 10 | 77.865% |
| H | 1.00794 u × 18 | 11.762% |
| O | 15.9994 u × 1 | 10.372% |
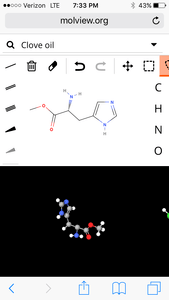
6. Clove
3D Model:
| Formula | C7H12ClN3O2 |
| Molecular weight | 205.642 u |
| Hydrogen bond donors | 3 |
| Hydrogen bond acceptors | 4 |
Percent composition
| C | 12.0107 u × 7 | 40.884% |
| H | 1.00794 u × 12 | 5.8817% |
| Cl | 35.453 u × 1 | 17.240% |
| N | 14.0067 u × 3 | 20.434% |
| O | 15.9994 u × 2 | 15.560% |
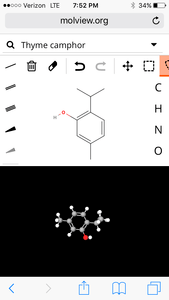
7. Thyme
molecular structure:
3D Model:
| Formula | C10H14O |
| Molecular weight | 150.221 u |
| Hydrogen bond donors | 1 |
| Hydrogen bond acceptors | 1 |
Percent composition
| C | 12.0107 u × 10 | 79.955% |
| H | 1.00794 u × 14 | 9.3938% |
| O | 15.9994 u × 1 | 10.651% |
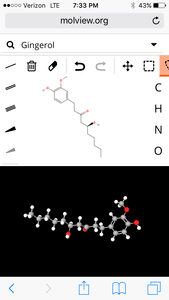
8. Ginger
molecular structure:
3d Model:
| Formula | C17H26O4 |
| Molecular weight | 294.391 u |
| Hydrogen bond donors | 2 |
| Hydrogen bond acceptors | 4 |
Percent composition
| C | 12.0107 u × 17 | 69.359% |
| H | 1.00794 u × 26 | 8.9021% |
| O | 15.9994 u × 4 | 21.739% |








wow so interesting!! The science behind essential oils opened my mind to why it is so good for us, thanks for sharing 🙂
Right?! I think it’s awesome to understand what we put on our bodies. A lot of us choose an oil or chemical substance that is not good for us without knowing. You’re welcome.